

So W is measuring how many energetically equivalent microstates a system can organize its energy into to get the same macrostate (or the system as we can observe it on a human scale).Īnother formula uses the second law of thermodynamics: ΔSuniv > 0, which put into words states that any spontaneous process increases the entropy of the universe (creates a positive change). A microstate is a particular way to order energy. Boltzmann’s formula uses a more mathematical approach to entropy by using W. The unit for entropy is the same as Boltzmann’s constant which means that entropy can also be understood as how many energy can be dispersed at a certain temperature since entropy is temperature dependent. One equation is Boltzmann’s equation: S = k*ln(W), where S is entropy (the usual variable for entropy), k is Boltzmann’s constant which is equal to the gas constant divided by Avogadro’s number which is approximately equal to 1.38 x 10^(-23) J/K, and W is the number of microstates which is a unitless quantity. There are several because entropy can be explained and used in a variety of ways.
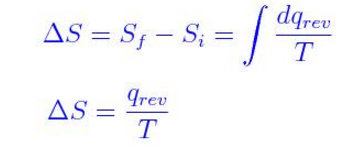
Since entropy is primarily dealing with energy, it’s intrinsically a thermodynamic property (there isn’t a non-thermodynamic entropy).Īs far as a formula for entropy, well there isn’t just one. First it’s helpful to properly define entropy, which is a measurement of how dispersed matter and energy are in a certain region at a particular temperature.


 0 kommentar(er)
0 kommentar(er)
